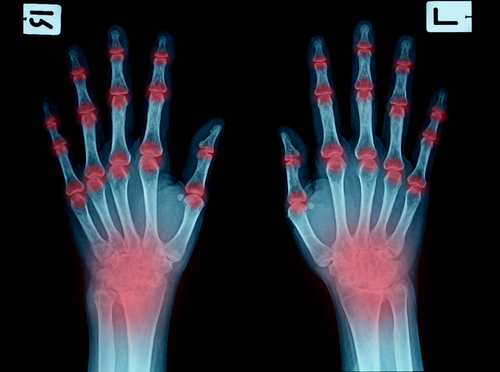
Immunology and Rheumatology
At the leading edge of immunology and rheumatology clinical research
Immunological, rheumatological and musculoskeletal diseases represent a significant burden for patients and society. PPD has extensive experience and targeted solutions to support development of programs addressing high unmet need across the disease areas. Sponsors face challenges in developing new medicines in a crowded and dynamic marketplace and PPD partners with them to guide every step along the way to delivering clear, high quality trial outcomes. This includes developing strategies to proactively address evolving needs such as diversity requirements in programs to meet FDA and EMA protocols.
PPD’s experience in the past five years:
Clinical trials managed
Global sites
Immunology and rheumatology patients
Lupus Center of Excellence
To enable drug developers to take on the challenges and opportunities in lupus clinical trials, the PPD clinical research business of Thermo Fisher Scientific has launched the Lupus Center of Excellence — bringing together a team of physician leaders, subject matter experts, operational experts, and data management professionals to operationalize and deliver high quality studies for pharmaceutical sponsors.
In recognition of the work being done in this space, Lee Simon, MD, joins us for a conversation that covers the history of drug development in lupus, current and novel endpoints, regulatory challenges, and strategies to ensure data integrity in lupus clinical trials including the use of proprietary data dashboards. Tune into the discussion.
A key to our success includes access to an extensive, proven network of investigative clinical trials sites built up through our experience in this therapeutic space.
Our comprehensive immunology and rheumatology expertise spans a broad range of indications and includes advanced treatment modality including rarer rheumatology and immunology
- Gout
- Hereditary angiodema
- Lupus – systemic and cutaneous lupus, lupus nephritis
- Osteoarthritis
- Osteochondromatosis
- Psoriatic arthritis
- Rheumatoid arthritis
- Systemic sclerosis
- Sjogren’s syndrome
- Vasculidites (giant cell arteritis, Behcet’s)
As immunological and rheumatology research expands treatment modalities beyond biological and targeted small molecules into mRNA technologies and cell and gene therapies, PPD is poised with extensive experience in these innovative treatment approaches. This includes a dedicated Cell & Gene Center of Excellence, which facilitates expertise-informed development of your asset no matter what technology or platform it employs.
To keep pace with the increase in rare disease research, our unit has expanded its dedicated resources. With experience conducting more than 70 rare immunology studies in the past 5 years, our team has deep knowledge of the key challenges such studies present — and how to overcome them. This knowledge enables us to guide drug developers through site identification, collaborate with KOLs to support enrollment and site engagement, and partner with patient advocacy groups to ensure the patient is at the heart of study designs.
Your partner for clinical development in lupus
Developing new therapies for Lupus comes with challenges. At PPD we understand those challenges. We share your passion for unlocking new ways to drive the development of your asset in the most effective manner possible. Leveraging cross-therapeutic expertise between immunology, dermatology and nephrology, we offer you a differentiated solution for Systemic and Cutaneous Lupus and Lupus Nephritis.
Our experts constantly innovate to enroll the right patients and improve data quality in Lupus clinical trials. Your trial needs subjects with the appropriate disease activity, but we partner with you to ensure that enrolled subjects reflect the diversity of the patient population. Tap into our global medical and operational expertise, high-quality sites and industry-leading enrollment rates.
Innovative approaches ensuring patient centric research
The COVID-19 pandemic accelerated development of technologies to enable digital and decentralized clinical trials, along with expectations from patients and physician communities for adopting these approaches to reduce patient burden. PPD is at the forefront of these new developments and is actively incorporating decentralized study approaches into sponsor’s immunology and rheumatology programs.
This includes services to enable a range of models, from a mix of in-home study and in-clinic visits (hybrid trials) to fully virtual site study. Bespoke design solutions are tailored to the specific study in hand – all with keeping the focus on the patient and reducing burden without compromising patient safety or data quality. Decentralized trials are a significant enhancement to patients with rare immunological disorders and are also finding their way into core rheumatology programs.
Understanding the importance of data quality in immunology clinical trials
Providing high quality data is core to robust clinical trial outcomes and, ultimately, regulatory dossiers supporting new drug registration. Reducing variability and ensuring the right patients are enrolled in your clinical trials is at the core of our work.
We have developed strategic partnerships with eCOA platform providers to better integrate full data inputs and facilitate patient eligibility review processes by an in-house data and medical expert team. This enables enrollment of the right target patients for your study along with dynamic, longitudinal data consistency review leading to more robust, consistent and reliable datasets.
Broad scientific and medical in-house immunology expertise
Our team of operational experts works hand-in-hand with our team of globally distributed in-house rheumatologists to deliver superior designs, strategies and data quality.

Jerry Green, MD FACR
Senior Medical Director, Pharmacovigilance

Sudhakar Sridharan, MD
Vice President, Medical and Scientific Strategy
“At the end of the day, the customer isn’t really ‘buying’ the company, they are buying a connection to the people who are represented at the table. [People] want partners who are personable, very approachable, who can answer questions in a way that lets them know they understand their needs and can come up with innovative solutions—and that they do all of that with energy and dedication.”