
Outsourcing from a Pharmacovigilance Perspective

Tina Bostic, executive director, pharmacovigilance, discusses PPD’s outsourcing strategy through expanding the global labor force.
Outsourcing case processing for marketed products continues to be the optimal method for many pharmaceutical and biotech companies seeking to lower expenses while maintaining regulatory requirements. However, finding and establishing trust with the right partner can be both challenging and costly. PPD understands these challenges and addresses them by continuing to develop strategies to support flexible costing models, sustain strong quality metrics and ensure on-time safety reporting. One of the ways that PPD aims to improve its outsourcing strategy is by continuing to expand its labor force around the globe, increasing capabilities to customize the right pharmacovigilance outsourcing model for our partners.
PPD recently acquired established pharmacovigilance CRO Vigi Medsafe, based in Hyderabad, India, with close to 200 experienced pharmacovigilance personnel. PPD selected Vigi Medsafe based on its leadership team’s commitment to ensuring quality work and their focus on employee development and retention. Vigi Medsafe boosts PPD’s ability to provide adaptable operational models for pharmacovigilance services, as Vigi Medsafe’s compliance for quality and on-time regulatory reporting is 99 percent. They deliver excellent quality results for case processing, aggregate report writing and signal detection services to their clients. PPD’s acquisition of Vigi Medsafe supports three objectives: It maintains strong quality, brings experienced pharmacovigilance staff and expands cost-effective global positioning to ensure business continuity of services.
Quality can be a concern when outsourcing case processing activities. PPD remedies this concern by using a robust global case quality process, which has proven to be effective. Our global case quality average is 99 percent. PPD’s safety tracking system (STS) is also an essential component, used for documenting, tracking and analyzing case quality scores, and works in conjunction with our global case quality process. Through the STS, management provides oversight of teams by identifying potential quality trends and rectifying any concerns through team communications, updated training and continued quality monitoring. All PPD locations utilize STS, ensuring consistent case processing management across locations.
Staff retention can also be a concern when outsourcing. At PPD, our corporate culture and Defining Principles are critical components to our success and ability to attract and retain key talent. Our process for minimizing employee turnover is selecting the right candidate for the position by matching long-term goals and motivations. Being dedicated to employee engagement, offering competitive benefit packages and providing career growth opportunities for staff are a few factors contributing to our consistently high retention rates.
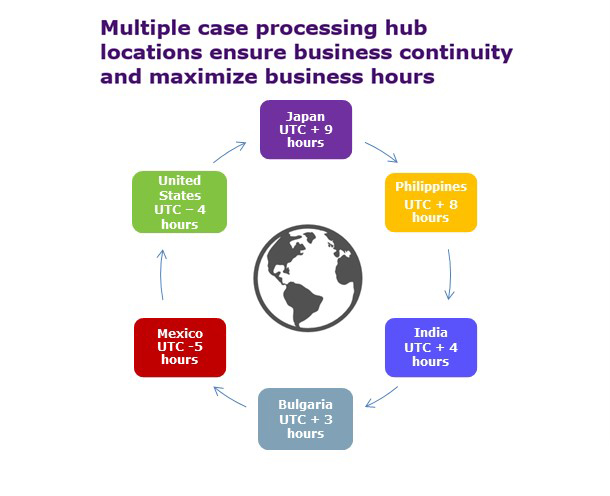
PPD also reaps many benefits from its global labor force. For large case volume projects, PPD recommends a two-location model to ensure business continuity. With multiple PPD locations, we can offer a variety of options for labor mix. A combination of support from India and the Philippines provides the most cost-effective solution for pharmacovigilance services. Additionally, providing a flexible operational delivery model to meet the demands of unexpected case volume from various sources—like patient support programs, literature and spontaneous reporting—is a critical part of maintaining quality and compliance. PPD can scale resources quickly to meet these demands. We do this through a successful recruitment and onboarding strategy to have new resources ready to begin taking on ad hoc requests in a relatively short time frame.
With strategic global locations and a submission compliance rate of more than 99 percent, PPD has delivered comprehensive, end-to-end pharmacovigilance services with proven solutions, exceptional quality and successful delivery to more than 575 biopharmaceutical, biotechnology and medical device companies. By further expanding our global labor force through the Vigi Medsafe acquisition and coupling it with our proven experience, PPD’s ability to provide customized solutions is enhanced without jeopardizing regulatory timelines or compromising scientific data quality.