Choosing Decentralized Clinical Trials
The rise of DCTs
The COVID-19 pandemic has challenged the viability of traditional clinical trials around the world. The use of decentralized clinical trial (DCT) options was on the rise before the pandemic, but the industry’s growing need to keep research moving forward and ensure the safety of clinicians and patients made the need for remote capabilities more important than ever. With new efficiencies produced by telemedicine, remote monitoring, direct-to-patient shipments, devices and more, these new options will remain an important attribute of clinical trials as we enter the digital age of research.
of respondents expect to run a hybrid/decentralized trial in 2022 (vs. 59% in 2021).
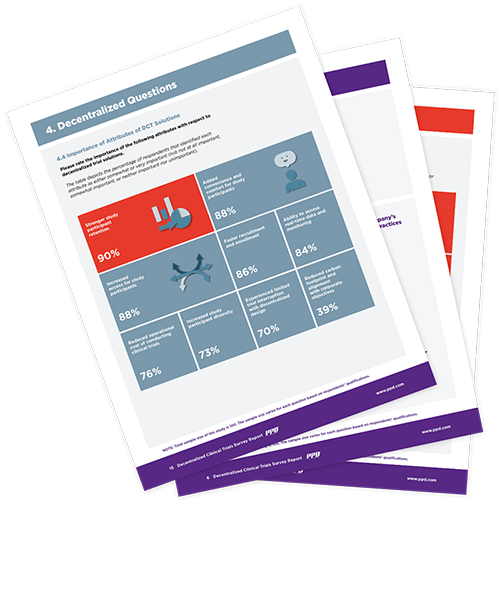
Why choose DCTs
As the clinical landscape continues to evolve, DCTs present opportunities to emphasize convenience, safety and flexibility, while continuing to pursue quality data and valuable research. Advantages include:
Environmental sustainability
PPD is dedicated to creating environmental innovations to better predict, measure and identify strategies to reduce the greenhouse gas emissions on clinical trials. We are partnering with stakeholders across the clinical trial spectrum to better understand how we can digitize, decentralize and modernize clinical research to make it more environmentally friendly.
Learn more about how we are creating a healthy planet to support healthy patients
Foster a sustainable future in clinical trials through decentralized trial models