
Digital and Decentralized
Meeting patients where they are
The PPD™ clinical research business of Thermo Fisher Scientific, designs digital and decentralized trial solutions that reduce barriers and keep patients engaged. Our services and tools meet participants where they are, whether it’s at home, at nearby clinics or on their devices. With remote trial services, mobile health care and virtual visits, we make participation easier and improve retention.
Additionally, we bring deep therapeutic expertise across major disease areas, including hematology, oncology, chronic conditions and emerging therapeutic areas such as immuno-oncology, cell and gene therapy, and rare diseases.

Expertly designed patient technology solutions
The PPD™ Digital implementation team leverages diverse industry experience and best practices to guide study design and oversee every step, from licensing and translations to testing and deployment, helping to implement streamlined, fit-for-purpose tools. By focusing on these areas, we unlock the full potential of digital patient technologies to support high-quality data collection and increase patient engagement and compliance.
Read article discussing digital selection and design considerations
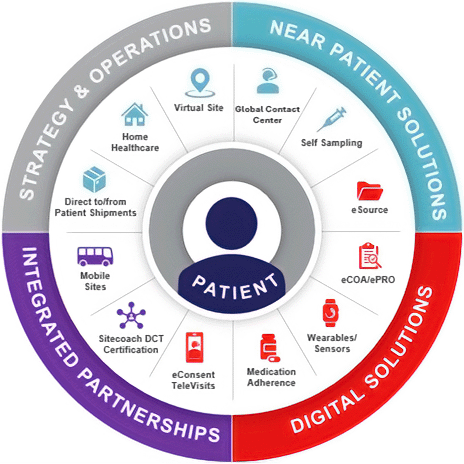
Digital and decentralized clinical trials (DCT) ecosystem
The PPD Digital and DCT ecosystem is a comprehensive assembly of strategic and operational approaches, near-patient and digital solutions, and integrated partnerships supporting trials that reduce patient burden, increase engagement, reach representative patient populations and cut trial timelines.
To continually advance our capabilities, we make ongoing investments in developing internal operational capabilities, integrating external businesses focused on patients and sites, and investing in innovative DCT trial platforms and service providers.

Enabling consistency through training and technology
We train and certify sites through SiteCoach and monitor trial adherence with our Preclarus™ analytics dashboard to support established protocols. Our technology suite includes eConsent, eCOA/ePRO, wearables, and medication tracking designed to enhance patient experience and data quality.
What it delivers
- Remote trials that reduce travel and boost participation
- DCT-certified site teams for hybrid and virtual study models
- Real-time engagement tracking and retention insights
- Participant-first tools that streamline onboarding and reduce dropouts
Improving protocol adherence with better access
Patient-friendly trial models are proven to reduce dropout rates and shorten study timelines. By minimizing travel and maximizing flexibility, we improve patient compliance and ensure study integrity across all phases of research.
Capturing cleaner, more timely data
With integrated digital tools and automated touchpoints, we improve data collection frequency and quality. Targeted outreach at key points ensures consistent input and supports adaptive trial designs.
Get in touch
Bring your trial to the patient. Talk to us about implementing digital and decentralized strategies that improve retention and make participation easier.