PPD Laboratory Services, Central Lab
Central lab services and quality data without compromise
Robust central lab services that offer an integrated, flexible, one-stop solution for the collection, management and analysis of lab and study data in the clinical trial ecosystem.
Clinical trial sponsors want accurate, reliable and fast data to successfully manage their clinical trials. It’s critical to engage a central lab that ensures consistent test methodologies, equipment and reporting standards, which drives standardization, accuracy and reliability of data across multiple study sites. Our global central lab helps enable informed decision-making, drive site excellence and address challenges that drug developers face. Through our award-winning drug development database solution, globally standardized clinical testing and centralized data visibility, our team helps customers make more informed decisions about their clinical trials.
We are dedicated to helping customers make faster, more informed decisions about their clinical trials through our award-winning drug development database solution, globally standardized clinical testing and centralized data visibility. Our central lab provides an integrated, flexible, one-stop solution for the collection, management and analysis of lab and study data, and an enhanced clinical focus.
Central Lab Overview
Access real-time laboratory data with our award-winning Preclarus™ lab solutions
When working with a large amount of lab data, powerful digital tools that provide data integration in real time allow for insights to help inform clinical decisions. But finding a lab service provider with the required expertise can be a challenge. Our award-winning Preclarus Lab solutions centralize and integrate data across labs to guide, accelerate and advance our customers’ development efforts.
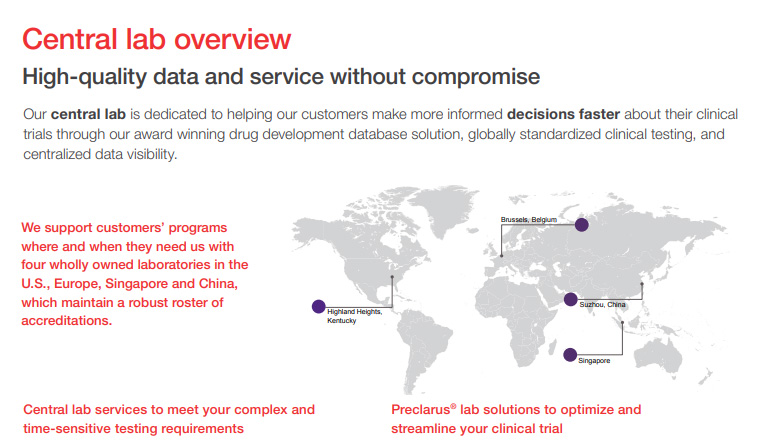
Global support for customers’ programs across the U.S., Europe and Asia
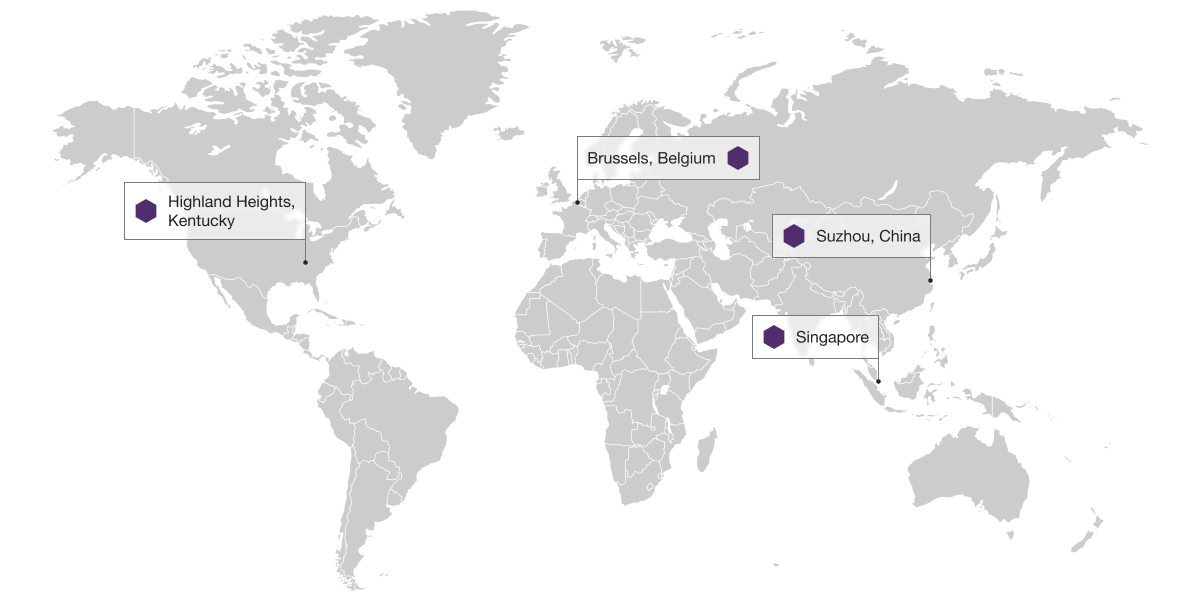
Our global network of laboratories has the ability to accommodate even the largest and most complex studies.
Take a virtual tour of our central lab in China
Learn more about the central lab in Suzhou, ChinaInvestment in people to make a difference
We maintain a people-first focus investing in our staff, fostering strong relationships with customers and driving initiatives to benefit patients. We believe that excellence is not a single action, but a habit built through regular training. We invest in our people by developing their careers. Our staff members are part of a global community, regardless of location or industry specialty. That’s why we created a collaborative training community and knowledge share program to invest in the future of our employees’ success.


Our commitment to project delivery and operational excellence
We know it is critical to provide strategic tools and systems that manage every aspect of clinical trials and emphasize patient-centricity. In addition to high-quality centralized laboratory services, we provide integrated data to project teams in real time to help inform decisions, especially for complex trials. Additionally, our robust IT team continually innovates and embraces the latest and greatest technologies.
Our global laboratory assay standardization survey (GLASS) drives harmonization and efficiency efforts across all study locations.
With Preclarus, results go directly into the database from our labs without a normalization/standardization step, providing data to study sites and project teams in real time.
- College of American Pathologists (CAP)
- Clinical Laboratory Improvement Amendments (CLIA)
- National Glycohemoglobin Standardization Program (NGSP)
- Quality Systems Essentials (QSE) as defined by the Clinical and Laboratory Standards Institute (CLSI)
Trending central lab content
Learn more about our central lab.
Learn more about PPD Laboratory services central lab.
To request a proposal or contact your local business development representative, please complete the form below.
If you are an investigator site and need immediate assistance, please call our central lab investigator support services hotline.
Americas: 1-859-781-8877
Europe: +32 2 275 2127 ext. 4300
Asia Pacific: +65 6594 6210