Bioanalytical Lab Experience and Expertise
Drive your drug development programs forward with an experienced partner
At every stage of your program, you deserve a dedicated partner that works with you to accelerate your development goals. For more than 30 years, the PPD™ Laboratory services Bioanalytical lab has partnered with clients on tens of thousands of molecules across various technologies. Ensure that your compounds reach the market efficiently and effectively by leveraging our industry-leading automation, regulatory expertise and global reach.
Discover how our extensive experience in biologics, ADC, biosimilars, small molecules, vaccines, biomarkers and more can work for you.
Biologics
Our bioanalytical lab has experience with more than 3,000 different biologics
PPD Laboratory services bioanalytical lab has been working with biologics since 1994 and our lab has experience working with all of the top 10 biosimilars in 2024. Our biologics development teams will work closely with you to understand the:
- Intended use of the assay
- Unique characteristics of the protein biotherapeutic
- Potential interferences
- Availability of reference materials and standards
- Current bioanalytical regulatory requirements
With this knowledge, our team designs and validates custom assays—or adapts existing assays—and quickly generates meaningful data to inform project decisions.
Immunochemistry for biologics
PPD Laboratory services bioanalytical lab offers comprehensive immunochemistry services to support pharmacokinetic (PK) and anti-drug antibody (ADA) safety assessments. We have broad biopharmaceutical experience with a variety of molecules including monoclonal and multivalent antibodies, antibody fragments, oligonucleotides, PEGylated proteins and antibody drug conjugates (ADCs).
Our experience includes:
- 878+ PK/PD ligand-binding methods
- 480+ immunogenicity/ADA assay
- 40+ plate-based neutralizing antibody assays
- 80+ oligomer/aptamer methods
Chromatography/mass spectrometry for biologics
Drug developers turn to us for our decades of experience in applying chromatography methods to the development and validation of LC-MS/MS analytical methods for biologics.
The bioanalytical lab’s experience applying LC-MS/MS and affinity capture LC-MS/MS methodologies to biologics includes:
- Extensive biologics experience, ranging spanning from small (1 kDA) therapeutic peptides to large (900 kDa IgM) proteins
- A wide range of sample preparation methods from simple protein precipitation to complex affinity capture enrichment techniques
- Development and validation of LC-MS/MS assays using surrogate peptides produced by proteolytic digestion for quantitation
- Deep experience with bioinformatic software for identification of proteotypic candidate surrogate peptides
- Semi-quantitative analysis of intact proteins (>10 kDa)
- DAR characterization of antibody-drug conjugates (ADCs) using high-resolution mass spectrometry (HRMS)
LC-MS/MS biologics capabilities
- Intact peptides, atypical peptides, monoclonal antibodies, Fab fragments and fusion products
- Antibody-drug conjugate (ADC) assays (preclinical and clinical):
- Total antibody
- Antibody-conjugated toxin (cleavable and non-cleavable linkers)
- Conjugated antibody
- Unconjugated toxin (auristatins, calicheamicins, pyrrolobenzodiazepines (PBD), tubulysins, maytansinoids)
- Multi-dimensional chromatography for the quantitation of biotherapeutics, peptides, proteins and biomarkers
- Preclinical and clinical matrices (fully regulated and nonregulated)
Our biomarker lab and our vaccine sciences lab are also deeply involved in the development of biologics and have significant experience with a wide variety of compounds, sample types and therapeutic applications.
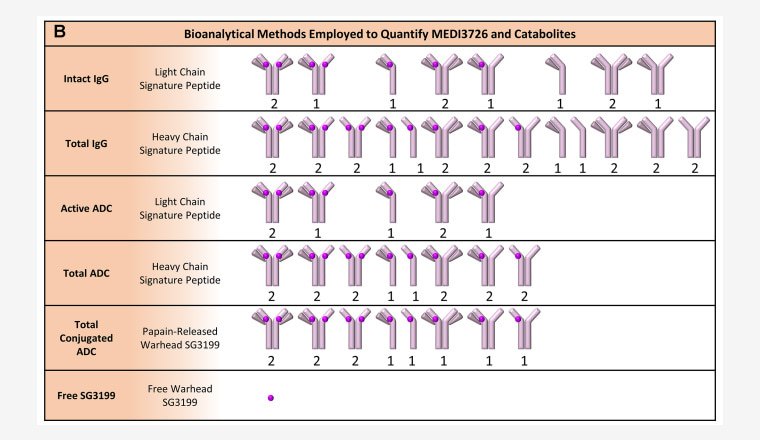
Comprehensive ADC development and testing
Our integrated bioanalytical laboratories work synergistically to accelerate your Antibody-Drug Conjugates (ADCs) programs from discovery through market.
At PPD Laboratory services, we provide a full spectrum of services for the development and testing of ADCs.
With extensive experience in immunochemistry and chromatography, we possess the expertise and flexibility to help you select the optimal platform for each assessment. Our integrated strategy supports ADC bioanalysis across all stages, including late-stage research, early development (IND enabling studies), and clinical development (Phase I, II, and III).
Immunogenicity assays
- 3-Tier LBA ADA Approach: screening, confirmatory & characterization, titer
- Plate- and cell-based Nab assays
PK assays
- Total (intact) ADC by LBA or hybrid LC-MS/MS
- Total Antibody by LBA or hybrid LC-MS/MS
- Free Payload (unconjugated toxin) by LC-MS/MS
R&D scientists supporting development and validations across immunochemistry and LCMS technologies

2024 Best Contract Research Award (CRO) World ADC Conference
Sample analysis
We have successfully supported 12 of the 15 FDA and/or EMA approved ADCs. Moreover, in the past 3 decades, we have supported the quantitation of conjugated molecules, antibodies, cleavable and non-cleavable linkers, and toxins for over 1,000 ADCs.
Our global network of dedicated team members will work closely with you to understand and support your unique development needs and programs.
chromatography assays to support quantitative analysis of the intact ADC, total antibody, and payload
LBA assays that support quantitative analysis and immunogenicity
cell-based NAb assays to support ADC immunogenicity
Biosimilar
We have supported more than 35 biosimilar programs destined for regulatory submission
Bringing a biosimilar to market requires extensive experience, broad technical capabilities and a deep understanding of the regulatory pathway. We understand the unique needs of a biosimilar drug relative to other biopharmaceutical compounds:
- Biosimilars must demonstrate comparable results (safety, purity, potency, stability and immunogenicity) to the innovator product and across product lots
- Due to the physicochemical attributes and functional activity of the biosimilar, assays developed for the innovator product may require adjustments and/or redevelopment and validation
- Biologics by nature are more variable than small molecules, making the analytical methods subject to variation across instruments, critical reagents, operators and even day-to-day and lab-to-lab differences
- Biosimilar development is complex and each project has specific needs
- Bioequivalence (BE) studies, anti-drug antibody (ADA) testing, and efficacy evaluations are crucial to explain changes from the innovator drug. Disease states and manufacturing changes can impact pharmacokinetic (PK) and ADA assessments. Manufacturing changes introduced between studies for scale-up or patent reasons can also have an effect on those assessments.
In addition to bioequivalence (BE) studies, anti-drug antibody (ADA) safety and efficacy testing are critical to the development of biosimilar to explain and justify changes from the innovator drug. Disease states can also affect pharmacokinetic (PK) and ADA assessments (even in Phase I studies). Manufacturing changes introduced between studies for scale-up or patent reasons can also have an effect on those assessments.
Competition in the biosimilar arena breeds extremely tight timelines. With our propriety method development and validation, regulatory expertise, and automation-driven efficiencies, we streamline the path to approval with high-quality testing that supports every stage of biosimilar development to accelerate time to market.
Our bioanalytical lab has supported more than 35 biosimilar programs in support of U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) submissions. We also have experience working with 17 of the top 20 and all of the top 10 Biosimilars in 2024.
We have an excellent FDA audit record, including more than 80 inspections. In addition, PPD Laboratory services is one of only a very few labs that has been audited for its work in support of biosimilar submissions.
Cell and gene therapy
PPD Laboratory services have worked with more than 300 cell and gene therapy assays.
Our bioanalytical lab has been working with these novel therapeutics since 1997. Our team has accumulated a wealth of experience in pharmacokinetic/pharmacodynamic (PK/PD), immunogenicity and molecular genomics, backed by extensive capabilities and strong regulatory expertise. At PPD Laboratory services, we support customers’ therapies through every stage of the drug development life cycle.
Our dedicated lab development team works closely with customers on their programs and supports a variety of analytes and delivery mechanisms:
- Oligonucleotides
- mRNA
- CAR-T therapy
- CRISPR/Cas9
- Enzyme replacement therapy
- Lipid nanoparticles
- Polymers
- Adeno-associated virus (AAV)
- Targeted delivery mechanisms
Expertise in cell and gene therapy
For the past 25 years, PPD Laboratory services has been building its expertise with these advanced therapies and has worked with more than 300 cell and gene therapy assays since 1997 to provide:
- Method development, transfer and validation and sample analysis following U.S. Food and Drug Administration (FDA) good laboratory practices (GLP) customized for cell and gene therapies
- Analytical expertise across a wide range of common and rare matrices including more than 20 years of experience with tissue processing and analyses
Extensive capabilities for cell and gene therapy development programs
PPD Laboratory services’ bioanalytical lab provides the state-of-the-art technology, cutting-edge instrumentation and high-quality services required for advanced cell and gene therapy programs in any phase of drug development. Our offerings include:
- Comprehensive immunochemistry, chromatographic, flow cytometry and molecular services to support preclinical studies and clinical trials Phases I-IV
- High throughput liquid handling and sample analysis
- Gene expression product analysis via ELISA, MSD, Gyros, Simoa and additional ligand binding platforms
- Wide-ranging molecular genomic services including qPCR, digital droplet PCR, Sanger sequencing and next-generation sequencing (NGS) to support preclinical through post-approval
- Anti-drug antibody and cell or plate-based neutralizing antibody assays for analyte, protein product and delivery vector
- Bioanalysis by ion-pair liquid chromatography coupled with tandem mass spectrometry (IP-LC-MS/MS) and IP-LC-UV
- CAR-T cell enumeration for cellular kinetics and immunophenotyping via flow cytometry
- View our CGT Capabilities Tech Sheet
- Technology selection guidance based upon sensitivity, selectivity, complexity, dynamic range, throughput and specific program needs
Generics
Our bioanalytical lab has helped develop more than 200 generic drugs, including six of the top 10 generics of 2018.
The PPD Laboratory service bioanalytical lab takes a proactive approach to method development for generic products by beginning early in the development life cycle. We support analysis for formulation development in addition to supporting pilot and pivotal studies.
Our bioanalytical lab’s generics teams have:
- Worked with more than 65 generic pharmaceutical companies around the world
- Supported more than 1,600 generic drug development studies
- Analyzed more than 2 million samples
- Conducted numerous first-to-file projects, and delivered results for many thousands of samples within a short period of time
The unique regulatory environment for generic pharmaceuticals requires an experienced development partner. The Abbreviated New Drug Application (ANDA) process has strict guidelines that require attention to detail and rapid turnaround to support Paragraph IV first-to-file submissions. We have successfully completed more than 50 onsite U.S. Federal Drug Administration (FDA) inspections for ANDA programs and our data has supported regulatory approvals through Medicines and Healthcare products Regulatory Agency (MHRA), European Medicines Agency (EMA), Brazil’s National Health Surveillance Agency (ANVISA), Health Canada and the FDA.
Small molecules
We have worked with more than 2,200 different small molecules compounds.
PPD Laboratory services’ bioanalytical lab has been contributing to the development of traditional small molecule pharmaceuticals for three decades. Our lab in Middleton, Wisconsin, is dedicated to small molecule studies, while our Richmond, Virginia, location supports small molecules and biologics. Together, our labs help us to offer compressive bioanalytical services for small molecule development including:
- Custom pharmacokinetic and pharmacodynamic (PK/PD) assay development, transfer and validation
- Sample analysis in multiple biological species and matrices
- Metabolite identification
- Mass spectrometry
- Liquid chromatography combined with mass spectrometry (LC-MS/MS)
- High resolution mass spectrometry (HRMS)
- High performance liquid chromatography (HPLC)
- Ultra-high-performance liquid chromatography (UHPLC)
- Paragraph IV first-to-file for generic drug development
Chromatography for small molecules
We have an extensive list of validated chromatographic assays to support bioequivalence, drug-drug interaction and other types of studies. Our lab is equipped with several instrumentation platforms including systems from SCIEX and Waters. This collection boasts more than 100 LC/MS systems, nearly a dozen multiplexing systems and approximately 30 UHPLC systems.
Our expertise includes:
- High Performance Liquid chromatography (HPLC, UHPLC), mass spectrometry (LC/MS) and multi-dimensional chromatography
- Derivatization and chiral assays
- Sample analysis in multiple biological species and matrices
- First-to-file generic pharmaceuticals experience
Veterinary pharmaceuticals
Accelerate your next program by leveraging more than 20 years of veterinary development and regulatory experience.
For more than 20 years, PPD Laboratory services bioanalytical lab has been conducting customized assay development and validation for both branded and generic veterinary pharmaceuticals. Our breadth of experience and capability spans small molecules, biologics and vaccines and involves a wide variety of disciplines including chromatography, ligand-binding, cell-based assays and molecular platforms.
Our development expertise and technical capabilities enable our veterinary clients to bend the cost and time curve of drug development to deliver life-changing therapies to the market as efficiently as possible.
Our capabilities include:
- Discovery analysis and formulation development studies to home in final product specifications
- Development of the study protocol and sample analysis plan to streamline the pre-concurrence process
- Customized/proprietary assay development and validation
- A wide variety of disciplines:
- Chromatography
- Ligand-binding
- Cell-based assays
- Molecular platforms
Our laboratories and instrumentation are ever-expanding and include multiple platforms for most assay types as well as high-throughput liquid handling systems.
Our dedicated animal health project managers leverage our experience and scientific expertise to ensure the success of each project. This experience includes:
- Small molecules, biologics and vaccines
- True GLP bioequivalence studies in plasma for large and small animals
- Tissue residue studies at concentrations below that of the residue marker
- Bioavailability studies to demonstrate true cause of therapeutic effect
- Quantitative testing of immune response and immunogenicity for vaccines
- The first lab to support the approval by the USDA of a monoclonal antibody in animals
- More than 30 years experience in bioanalytical testing and more than 20 years in animal health
All testing for veterinary pharmaceuticals, from pilot to pivotal studies, are conducted to true GLP standards.
Important regulatory exposure
The success of your development project relies on attention to detail and regulatory compliance. PPD Laboratory services bioanalytical lab has an excellent working relationship with the U.S. Food and Drug Administration Center for Veterinary Medicine (FDA-CVM) and several international health and drug safety agencies. We were one of the first labs to submit electronic hyperlinked reports to the FDA-CVM. This new approach has virtually eliminated “incompletes” and has streamlined the approval process for our clients.
PPD Laboratory services does not maintain vivariums or perform testing of any kind on animals.
PPD Laboratory services bioanalytical lab thought leadership series
Watch as experts from our bioanalytical, biomarker and vaccine sciences labs in Richmond, Virginia, take you through trending topics of the bioanalytical landscape ranging from CAR-T cell enumeration to vaccine immunogenicity testing.
Related resources
Learn more about our bioanalytical experience and expertise.
Learn more about PPD Laboratory services bioanalytical lab
To request a proposal or contact your local business development representative, please complete the form below.