
Category: Regulatory & Safety


Key Considerations for Successfully Planning a Submission
Read More


Prepare Now for EU Clinical Trial Regulation Go-Live Date
Read More
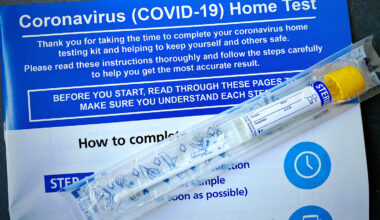
Devices/Diagnostics: Emergency Use Authorizations During the COVID-19 Pandemic
Read More


REMS: To Outsource or Not to Outsource?
Read More


New and Emerging Regulatory Guidance for Cell Therapy Product Development
Read More


The Critical Need for Pregnancy Registry Studies and Robust Safety Monitoring in COVID-19 Vaccines
Read More


Predicting Extractables & Leachables Amounts Using Mathematical Modeling
Read More


Moderna Announces FDA Authorization of Moderna COVID-19 Vaccine in U.S.
Read More


Time to Act is Now: Paediatrics Medicines Development in the UK
Read More