Preclarus Lab Solutions: Integrated Real-Time Lab Data for Clinical Trials
Explore the importance of robust central lab solutions that offer an integrated, flexible, one-stop solution for the collection, management and analysis of lab and study data in the clinical trial ecosystem.
A central lab is a specialized facility providing centralized services including laboratory tests and analyses for clinical studies and clinical care. These labs are essential for standardizing, ensuring accuracy and maintaining data reliability across multiple study sites. They provide consistency in test methodologies, equipment and reporting standards.
Accurate and reliable lab data are vital for any clinical trial. Real-time access to clean data enhances operational efficiency and helps clinicians and sites make informed decisions for patients. The vast amount of data generated in clinical studies requires powerful, dynamic digital tools for real-time data integration, providing insights to help inform clinical decisions.
Strategic tools and systems that effectively manage all aspects of clinical trials help address challenges, enable informed decision-making, and drive site excellence. Today’s central lab solutions often integrate advanced technologies and digital tools to streamline processes and ensure seamless data flow. They also handle logistical aspects like sample collection, transportation and storage, while adhering to stringent regulatory standards.
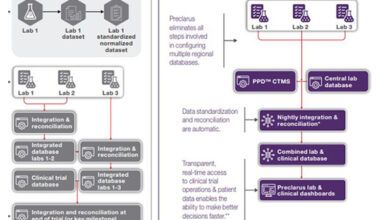
In this article we will review the importance of using central lab solutions that provide an integrated, flexible solution that collects, manages and analyzes lab and study data in clinical trials. Preclarus™ Lab Solutions offer a cutting-edge data and analytics platform with real-time access to study and lab data. A single global database — enabling study teams to access information within a study all at one time, in real time — provides transparent reporting and analytic capabilities to drive clinical decision-making and address challenges. A proprietary, web-based enterprise and information management system for the central lab provides a fast and accurate study startup and uses data visualizations and interactive reporting tools that turn data into actionable intelligence.
Lab data challenges that drug developers face today
Lab data management challenges can have significant implications for data quality, research outcomes and patient care. Customers want their central lab to acquire and integrate lab data in real time. While lab test data is the primary focus for most central labs, there may be operational data that is lacking. For instance, sample testing involves more than simply receiving a sample, testing it and producing a result. There are many moving parts to consider, including a central database, managing the ordering of test kits, shipping kits to sites, kits being translated into samples, tracking samples as they’re sent back to the lab, sample analysis, results reporting and analysis, and sharing data with clinicians. This is difficult to do from a complete data perspective without project visibility in real time. Also, even if the operational data is effectively captured, it is crucial to surface and analyze it to create efficiencies that save time and money.
Many customers struggle to visualize the real-time progress of their studies without tools that provide transparency and details of each step of the process, including sample chain of custody, outstanding queries (site-level clarification of data discrepancies), kits, and supplies used. Additionally, the complexity of clinical trials has increased, necessitating flexible systems that can adapt, maintain efficiency, and accelerate production.
The development of Preclarus was guided by the identification of key industry challenges and the creation of solutions to them within a single platform.
Preclarus: The lab data solution from PPD Laboratory services
More than 15 years ago, our central lab team understood that tactical operational data was lacking across the industry and set out to develop the right clinical trial tools that generate actionable data. A single global database is critical for multi-center trials that are often conducted around the world. Multi-center trials operate in the same way and face similar challenges, yet it is important to have visibility to all of them simultaneously. This is what led to the development of Preclarus Lab Solutions, which provide that visibility and enable study teams to access information within a study at one time, in real time. Instead of several databases, there is one database to work with, allowing for faster setup speed.
Study database setup is also faster because Preclarus is a web-based application, making it broadly usable and user-friendly without a specialized data or IT team for programming. Protocols are easily transferred into the database through a series of modules in the web-based application, allowing for self-editing. As a single global database, it can be programmed from anywhere in the world with web access.
Some of the features and solutions that Preclarus offers include:
Sample chain of custody
The sample chain of custody is an important challenge in industry. Tracking samples from the point of collection through the entire life cycle can be difficult. This includes collecting details such as information about the airway courier bill, the different modes of transport involved in getting the sample to a lab, and identifying the type of lab (i.e., central versus non-central lab). It is therefore critical to be able to track the chain of custody through the entire journey. Preclarus tracks the chain of custody of millions of samples in real time.
Real-time error checking
Identifying and correcting errors is also a significant issue. Preclarus was designed to help prevent mistakes at the point of entry through real-time error checking. We were familiar with the common types of errors that can happen, so the goal was to integrate real-time error checking to guide users to enter information — simple and complex — correctly the first time. This includes simple tasks like inputting correct dates as well as more complex data measures and processes.
Meeting study timelines
Cleaning up data after errors have occurred downstream (i.e., from a central lab to third-party labs, biorepositories, etc.) can be complicated, tedious and time-consuming. Preclarus provides real-time error checking—which can prevent issues due to the data being continuously monitored and cleaned—leading to clean, error-free data at interim and final database locks, helping adherence to timelines. Error prevention is significant for studies that have hundreds or thousands of patients and visits.
Client-centered usability
There are many different types of studies, disease states and timelines to consider. Preclarus was designed to handle the different and changing components simultaneously within the system. The design team also continually invests efforts in the functionality of the system. We take customer feedback and information and continually make improvements based on needs, in order to solve the most important problems customers face.
Querying
Site level clarification of data discrepancies — queries — is critical to trial execution. For example, if mistakes are made, the information is sent to the site and also to the client (Sponsor) or a clinical research organization (CRO) partner that is involved to ask about the errors and see how they can be rectified.
The goal is to prevent errors and other issues, not just correct them. The tool guides users as they enter data through inbuilt real time quality control checks and guidance when there are multiple options available.
Data and tracking of supplies such as kits, including how they can be ordered, is also information that is housed within Preclarus. It has query engines that allow users to locate supplies for purchasing and other activities, like an online shopping tool. An agent is also available to help resolve issues. Importantly, the platform’s data mining is powerful and monitors operational issues including errors. In the end, the goal is to deliver clean, actionable data to help clinicians implement more robust monitoring plans.
Lab data collection and data quality
Preclarus endeavors to provide data on every measure and element in a trial. This includes principal data that is sent to investigators in a timely manner so that they can make important clinical decisions for their patients.
Strong data query capabilities provide higher data quality. Evaluating data across studies conducted involving thousands of sites and millions of samples to compare the use of Preclarus with older methodologies, such as paper-based methods, they found a ~67% reduction in overall query rate with Preclarus. Clients have shared through their own analysis that they found an approximate 80% decrease in queries with Preclarus versus traditional paper methods.
Additionally, establishing a robust chain of custody can decrease the number of lost samples. This entails a list of samples, including samples in storage that are not in an active mode of transport or processing.
Implementing feedback for success
By choosing Preclarus, organizations are not just adopting technology — they are partnering with a system that has been meticulously crafted to support their success. Our team has received years of customer feedback, and the user response continues to be positive. The COVID-19 pandemic changed trial procedures and led to the usage of different technologies to enable remote work; our team then noticed a significant shift toward the acceptance of electronic processes. Study sites have since shared that they appreciate no longer having to use paper. Additionally, they have noticed fewer queries, which saves them a lot of time. The tool has significant value and relevance in the current clinical trials landscape.
Because of our focus on being the central lab of choice for sites, we make updates based on user/site requests for specific additional functionalities. This ensures that our customers have a complete tool set they can use to help effectively manage their clinical trial.
Outlook: Digital tools and technologies for an evolving clinical trial landscape
Managing the life cycle of laboratory data, from collection to archiving or disposal, while ensuring it remains useful and accessible, is difficult and complex.
In addition to a technological solution, Preclarus is a comprehensive system built on years of experience and a deep understanding of clinical trials. In an industry where precision, reliability and efficiency are paramount, the unique features of Preclarus Lab Solutions meet the challenges of today’s complex clinical trial landscape.
The rapid evolution of technology through Preclarus Lab Solutions in data management and analysis means laboratories can leverage technological tools to continually adapt and upgrade their process and effectively deliver trial components from sites to a patient’s home.
We will continue to focus on digital innovation to meet the needs of our customers as part of the most digitally forward provider of CRO services in the industry. We aspire to bring digital, data, analytics and AI innovations to help our customers in their missions to bring scientific innovations to patients faster and better than before.
Unique technological innovations from Preclarus Lab Solutions
Preclarus is not just about keeping up with industry standards — it’s about setting them. The system includes several unique features that competitors struggle to match:
- Investigator site portal: Streamlines the process for site investigators, ensuring seamless communication and data management.
- Sponsor facing portal: Provides sponsors with real-time access to trial data, enhancing transparency and decision-making.
- Mobile applications: Facilitates on-the-go access to critical trial information, improving responsiveness and flexibility.
- Quality control at the point of care: Offers automatic quality control checks at the point of scanning a barcode, which is crucial for maintaining data integrity. Competitors often lack this immediate QC system, leading to potential quality issues.
These innovations enhance the entire clinical trial process, making it more efficient and reliable.
Learn more about our comprehensive data solution that consolidates and standardizes data from multiple sources to provide study teams with real-time access to all clinical trial operations.
Recommended for you