Archives: Resources
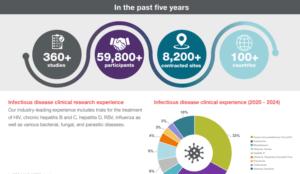
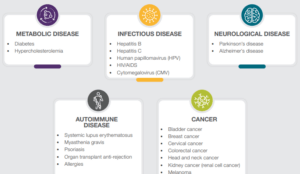
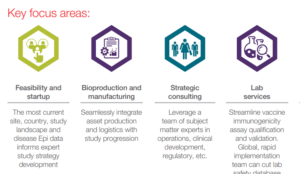
Your experienced infectious disease partner
Our industry-leading experience includes clinical trials for the treatment of HIV, chronic hepatitis B and C, hepatitis D, RSV, influenza as well as various bacterial, fungal, and parasitic diseases.
Diverse analytical approaches for complex oligonucleotides
This white paper explores the complexities of manufacturing, analysis, and regulatory requirements, offering insights into improving drug delivery, target specificity, stability, and compliance.
FSP outsourcing trends and opportunities
Experts discuss the latest trends and strategies in FSP partnerships, addressing drug development challenges and ensuring on-time, on-budget delivery.
Precision medicine’s next frontier: Unlocking the power of biospecimens
Discover how linking biospecimens with patient data and AI-driven innovations is transforming translational medicine.
FSP Pharmacovigilance safety reporting in clinical trials
Gain insights into regulatory challenges, compliance, country-specific nuances, and electronic reporting.
Driving acceleration in oncology drug development and clinical trials
Hear from experts on leveraging strategic collaboration to enhance speed and agility and gain actionable strategies with Thermo Fisher Scientific’s Accelerator™ Drug Development 360º solutions.
How to deliver on the potential of therapeutic vaccines
The explosive growth of the therapeutic vaccines global market presents immense opportunity for drug developers. Learn about the evolving vaccine landscape and what considerations must be addressed for successful therapeutic vaccine development.
Pandemic preparedness and rapid response overview
A new delivery model for pandemic preparedness and rapid response