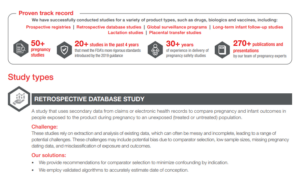
Archives: Resources
Registries and Real-world Evidence
Customizable registry solutions for actionable insights, tailored study design, and regulatory-grade data to accelerate development.
Peri- and Post-approval Safety and Real-world Insights
Learn how we optimize post-authorization safety obligations with a holistic, collaborative, and custom study design approach.
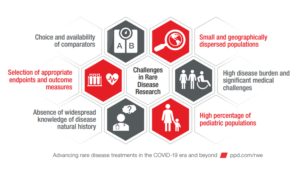
Advancing Rare Disease Treatments in the COVID-19 Era and Beyond
Read our white paper on the continued interest in developing treatments for rare diseases.
Pediatric Drug Development: Trends in the EU
A discussion on Pediatric Investigation Plans (PIPs), a 10-year report on the impact of the Regulation, and pediatric clinical trials.
Detailed data analytics improve performance metrics
Clinical care settings rely on teams with many layers functioning at their optimum capacity.
Increasing patient recruitment and administrative efficiences
Efficient clinical research mandates the implementation of precise processes to optimize outcomes and results.
Supporting optimized management for both scientific research and clinical practice
Across the health care ecosystem, preclinical research, clinical trials and clinical medicine coverage to deliver better care to patients.
A connected value story supported by strong evidence
Scientists, statisticians, modelers, and consultants create a unified value story and body of evidence.