Archives: Resources
PPD | Article
Beat the clock in clinical development functions
Discover how an FSP model can optimize a follow-the-sun approach in this Pharmaceutical Outsourcing article
PPD | Article
Five keys to successfully implement functional service partnerships
Read more about how FSP models help meet demanding timelines
PPD | White Paper
Outsourcing lifecycle maintenance to a dedicated FSP regulatory affairs partner
Ensure adherence to regulations and the continuous supply of drugs to target markets
PPD | White Paper
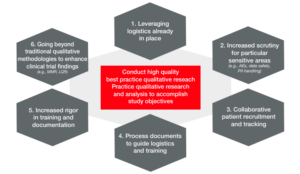
Ensuring effective regulatory intelligence in pharmacovigilance
Discover key pharmacovigilance regulatory intelligence capabilities for accuracy and cost-effectiveness
PPD | Webinar
Long-term follow-up considerations for cell and gene therapies
Experts share insights when considering long-term follow-up studies in the CGT space
PPD | Webinar
The 2024 FSP trends webinar
Experts discuss the future of FSP strategies and models
PPD | White Paper
Establishing a bespoke outsourcing arrangement with hybrid FSP/FSO partnerships
Optimizing your clinical trial operations with a hybrid FSP/FSO model
PPD | Video
Unique considerations in pediatric clinical research
Explore the intricate challenges of pediatric clinical research with our rare disease and pediatric experts.