Archives: Resources
Biotech Medical Information: Ensuring a Successful Product Launch
Discover how the right medical information approach can shape the success of your product launch.
Balancing Quality by Design
Explore Quality by Design (QbD) for efficient clinical trials. Discover how proactive design, AI tools, and sponsor-CRO collaboration ensure quality.
Accelerator™ Drug Development
Transforming the pharmaceutical value chain for emerging biotechs and large pharmas. Integrating drug development, manufacturing, and clinical research enhances efficiency and accelerates time to market.
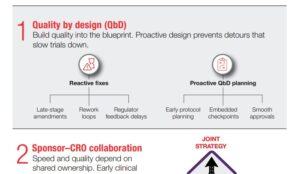
Accelerated Clinical Trials Through Quality by Design Principles
Uncover practical strategies for achieving accelerated clinical trials without sacrificing quality or compliance.
Obesity Clinical Trials: Beyond BMI
Watch our webinar to learn how evolving definitions and diagnostic criteria are reshaping obesity clinical trials.
An experienced respiratory partner
Global experience planning, implementing and delivering successful respiratory clinical trials.
Accelerate your clinical trial with expertise in breast cancer drug development
Learn more about our breast cancer capabilities.