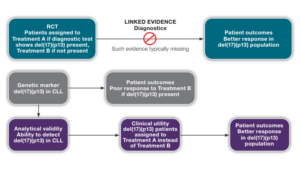
Archives: Resources
Reimbursement Landscape for NGS in Oncology in Australia, Canada and the United States
Gain insight into the usefulness of “next-generation sequencing” (NGS) in guiding treatment decisions for patients with cancer.
Reshaping the Future of Consumer Health through Decentralized and Digitally Enabled Strategies
This paper explores the unique challenges in consumer health research and highlights the benefits of using DCT strategies and digital technologies to improve data collection quality, speed, and relevance.
Preclarus Central Lab Database Infographic
Real-time data integration and query reconciliation can help you get your product to market quicker. Our proprietary interface has been integrating lab and clinical data in real time for more than 10 years. Trust our experienced team to deliver high-quality, integrated, study-wide data so you can make better decisions faster.
Extensive Multidisciplinary Experience in Delivering End-to-end REMS Solutions
Explore our comprehensive expertise in REMS programs.
GMP Lab Nitrosamines Analysis Case Study
In this case study, PPD® Laboratories GMP lab shares insight on how they completed method development, validation and testing to screen and quantitate NDMA in a drug product for a large pharmaceutical customer.
GMP Customized Assay Development and Complex Testing for Two Inhaled Drugs
In this case study, PPD® Laboratories shares insight on how their GMP lab developed customized assays and conducted complex testing for two different inhaled drug-device combination products.