
Natural History Studies in Rare Diseases and Genetic Biomarkers

Andrew Bevan, director, project management, peri- and post-approval operations, Evidera; Moira Ringo, senior consultant, real-world evidence, Evidera; Leona C. Fitzgerald, senior director, regulatory affairs, PPD; Fiona Kearney, senior director, project management, peri- and post-approval operations, Evidera; and Delphine Saragoussi, research scientist, real-world evidence, Evidera, discuss new guidance on rare diseases.
The increasing development of orphan drugs and precision medicine has led to novel needs in terms of real-world evidence generation. A key area recently highlighted in the FDA’s updated draft guidance on rare diseases is the recommendation of natural history studies to better characterize patient populations and delineate target populations. Natural history studies are epidemiological studies that focus on describing the frequency, features and evolution of a disease by collecting real-world data from groups of patients suffering from this disease. These studies are often performed early in the clinical development process to support and guide the design of clinical trial and drug development studies.
In the last few years, natural history studies have started to include genetic testing to describe specific genetic profiles as part of the features of the patient population, or as a screening criterion to identify the target population. The introduction of genetic testing within a fully non-interventional setting poses regulatory and ethical issues that are addressed differently by approval bodies (ethics committees, regulatory agencies, privacy committees and others) across the globe, highlighting the need for ongoing interpretation of the current regulations.
Increased focus on rare diseases and precision medicine
The recent wave of new product introductions in rare diseases and precision medicine (including targeted oncology indications) has allowed improved outcomes for patients who would otherwise face grim prognoses. However, health system budgets have not necessarily adjusted to the high prices and increasing number of available therapies in these categories. This has led to a need for “triage” strategies that allow payers to select therapies that do the most good for the least utilization and cost. Consequently, payers manage the financial impact by restricting eligibility and reimbursement only to patients who are likely to have a significant benefit over standard of care.
The role of natural history studies in the drug development strategy
The increased attention from biopharmaceutical companies and payers on rare disease and orphan drugs means there is a greater need to be able to accurately define the profile, characteristics and disease outcomes of the target patient populations. This is where natural history studies play a key role for both stakeholders.
On one hand, natural history studies can inform clinical product development by:
- Providing better insights into disease characteristics, patient populations, and identification of disease subtypes;
- Identifying the most sensitive and relevant endpoints or the optimal duration of follow-up;
- Identifying patients eligible for clinical trials; and
- Serving as an historical comparator in case of single arm trials.
On the other hand, natural history studies help payers “triage” care to patients most likely to benefit from therapies by:
- Assessing disease burden in real-world clinical practice under standard of care;
- Identifying and describing sub-types of a disease that have a higher burden;
- Identifying patient sub-populations who are less or more likely to respond to current therapies; and
- Identifying patient sub-populations that are likely to have the greatest benefit versus risk with new therapies.
The emergence of genetic biomarkers in real-world evidence generation
Biomarkers are an integral part of natural history studies for rare disease and advanced oncology therapies since many of these diseases are caused by inherited genetic mutations. The number and specific type of mutations can be highly predictive of disease severity and treatment response. The FDA now considers biomarker identification or validation as a full part of natural history studies in rare diseases. This introduces a new paradigm into the regulatory and operational aspects of running natural history studies.
Genetic testing
Currently, there is no consensus on the definition of genetic testing, despite the many organizations and governments who have voiced a desire for such an agreed upon definition.
The figure below visually summarizes the relevance and connection of the elements that drive the regulatory and ethics pathways, showing how study objectives, geography and genetic testing all play a role in the type of studies required for treatments for rare diseases.
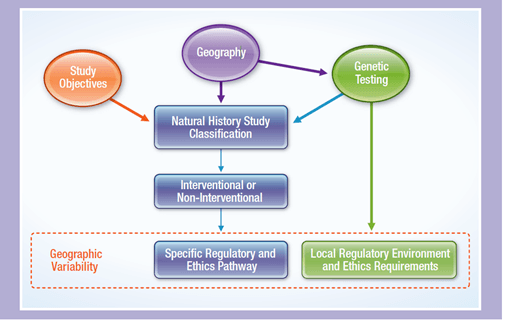
Drivers of the regulatory and ethics pathways
The emergence of new therapies focusing on rare diseases and targeted oncology indications leads to new needs in terms of real-world evidence generation, with the development of a new kind of natural history study that includes biomarker testing. These new needs challenge the regulatory framework that was initially shaped by clinical trials and traditional non-interventional studies, and this new situation translates into a diverse and moving regulatory environment for this new type of study. A timely and integrated multidisciplinary approach based on consistent strategic, scientific and operational considerations allows for anticipation of challenges and planning of preparatory steps for successful implementation of such studies.
For more information, read the entire white paper on this topic.