Harnessing Data to Drive Enrollment and Site Activation
Access to high performance sites
You want the best-performing sites with a patient population representative of your target market. You want experienced staff, down to the site level. And, you want complete data to inform early decisions.
These aspects of site selection and enrollment are often promised, but are they attainable? Yes. They’re possible with the PPD™ clinical research business of Thermo Fisher Scientific.
Going beyond traditional CRO-site models, we leverage the sites we own and the our Select network, our top highest-performing sites based on past performance. Our Select sites leverage streamlined feasibility processes to achieve 30% faster startup, while our Accelerated Enrollment Solutions (AES) sites achieve 2x faster enrollment than industry average.
Achieving enrollment 2x faster than the industry average
Big data. Small data. De-identified data. Anonymized data.
Thousands of data points feed your patient engagement and site selection processes. The challenge is finding meaningful metrics that drive actionable insights and optimal trial outcomes.
We own the most robust form of patient data: fully identified information on 100 million households of opted-in patients who are instantly accessible for trial recruitment, patient feedback and patient reported outcomes.
Fully identified patient data distinguishes us as a leader in data-driven feasibility, patient recruitment and retention
We triangulate data from a variety of sources to:
- Assess study feasibility
- Assess standard of care
- Optimize protocol and trial
- Identify optimal sites
- Recruit qualified patients
Our patient data are fully identified
Anonymized data from medical claims, pharmacy and other large datasets
Anonymized electronic medical record (EMR) data with only disease-specific information, patients not identified
Proprietary database of 100 million households of opted in patients with self-reported diseases and clinical trial histories
Site and patient visualization tool
Our proprietary site and patient visualization tool combines fully-identified patient data with site and study data to pinpoint the optimal mix of countries and sites for your study and your patient population.
- Fully automated cloud tool aggregates, integrates and geocodes data
- Predictive analytics compare study scenarios and optimize site selection
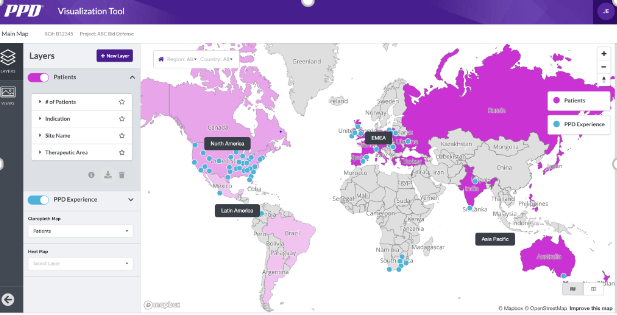
Data-driven feasibility triangulates data from site, patient and landscape sources
We combine our proprietary patient data with external sources to provide the most comprehensive view of patient, site, industry and investigator data to optimize patient enrollment and site selection.

Full enrollment, locally and globally
Data are the currency of clinical trials. The better the data, the more precisely you can plan, budget and execute.
Not relegated to a single source, we leverage de-identified, pre-identified and fully identified patient data to determine feasibility and accurately predict true enrollment.
Looking beyond your own trial’s footprint, we evaluate local competitive environments to identify concurrent trials, epidemiologic trends and other key determinants of recruitment success in each geographic region.
When it’s time to scale up, our site network — and site-level infrastructure — is built for immediacy and flexibility globally.
Partner with us
We can enroll more patients at fewer sites, while also ensuring a demographically rich subject base. While strengthening site onboarding and contracting consistency, our centralized trial oversight will reinforce data and process harmony.
If you are developing a protocol, it’s time to reach out to us. Holistic strategies enable us to assess and enhance site selection and recruitment strategies based on protocol design. We can even quantify potential trial performance based on nuanced changes to your protocol design.


Engage with us early, and we’ll expertly guide study design by quantifying expected trial performance based on a finely tuned protocol while mitigating the need for protocol amendments.”