
Strategically Selecting the Right Outsourcing Model for Drug Development — Considerations for Emerging Biotech

Elisha Talley-Roithner, senior vice president and global head of operations, PPD™ Biotech solutions, and Timothy King, executive director, PPD™ FSP solutions, discuss selecting the right outsourcing model for drug development.
There are several outsourcing models that have been created over the years to address drug development service needs. When or how companies decide on which outsourcing model is ideal for them will depend on a number of factors unique to the company. Cost savings is likely the main motivator for large pharma companies. Whereas for biotech, it might be growth.
Large pharma were the early adopters for functional service partnership (FSP) models, such as full time employee (FTE)-based engagements where vendor staff is 100% dedicated to a client and working within the client’s systems and standard operating procedures, historically called staff augmentation. However, FSP models have matured significantly, and smaller biotech companies are now able to take advantage of more flexible and diverse outsourcing models, to drive quality, cost controls, better efficiencies and faster delivery.
When thinking about how to best engage with potential vendors — it is helpful to start with two questions:
1) Do you prefer to have people fully dedicated to integrate with your own team members; or do you prefer to pay by the unit or task (database built, monitoring visit complete, SAE case processed, etc.), understanding that this means the staff may not be dedicated to you?
2) Do you need vendor staff to work in your systems (CTMS, eTMF, EDC, IXRS, EMS, etc.) and SOPs, or do you need the vendors to use their own systems and SOPs?
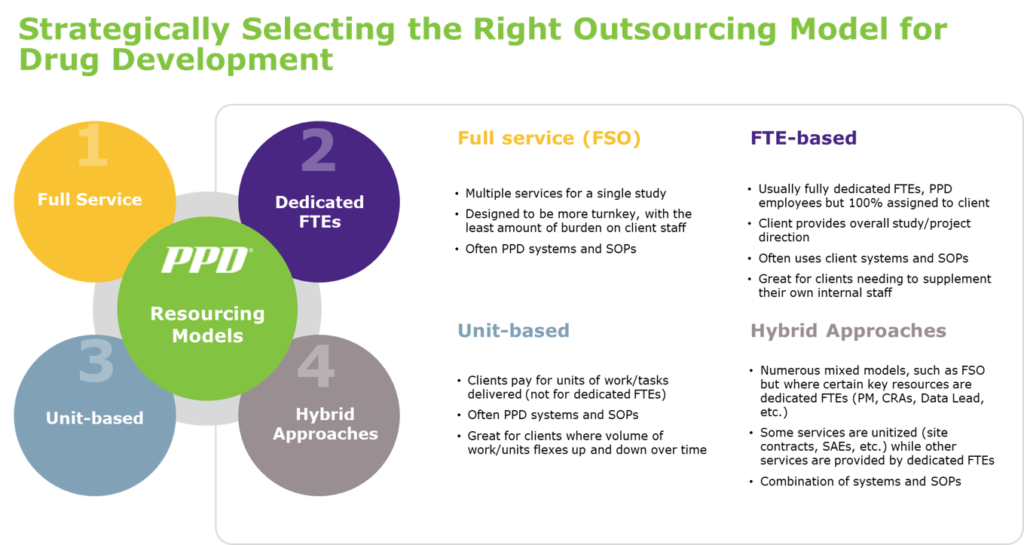
In today’s drug development environment, companies are not limited by having to commit to one model — allowing you to select a model that best fits your needs. Working with a vendor can help you confidently transition across the spectrum to simplify your resourcing model to drive operating efficiencies, provide high-quality services and reduce your study program costs.
Biotechs sometimes have needs very similar to large pharma, requiring dozens or hundreds of dedicated FTEs all around the world to support multiple protocols in client systems. Conversely, an organization that is in early stages of development and growing may require the full service outsourcing option to execute a clinical trial, as well as a few key positions to augment their existing team as the company grows.
Let’s walk through some examples:
Client A: A very small client may just need a few FTEs until they are ready to completely outsource a whole trial or trials. This client may benefit from an FTE-based resourcing model.
Client B: A client who has recently insourced their first oncology compound but does not have much internal oncology expertise may start off with full service outsourcing (FSO) for their first trial or trials, and then move to FSP as their internal expertise grows. This client may benefit from a hybrid model.
Client C: A rapidly growing biotech who needed a diverse set of outsourcing options to staff for internal functions, such as pharmacovigilance and data management. This client may benefit from a united-based outsourcing model.
From large pharma to biotech, choosing mixed or hybrid models across portfolios in development is a growing outsourcing option. With this approach, incorporating parts of FSP and FSO in a bespoke manner, helps drive quality, better cost controls, more efficiencies and faster delivery.