
Estimands in Oncology Trials
In this blog post, our cross-functional estimands working group explores how the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use’s (ICH) E9 addendum impacts oncology clinical trials.
The first draft of the estimands addendum was released for consultation in August 2017 and finalized by ICH E9 (R1) in November 2019. At their core, estimands comprise a novel framework that clarifies what it is we are trying to estimate about efficacy, safety or pharmacokinetics in clinical study protocols.
There may be misconceptions that an estimand is merely new terminology for the work we already do in evaluating treatment effect, but it is more than that. The estimand framework is a systematic, novel approach that is recommended by regulatory guidance, aiming to drive transparency and alignment of trial objectives to key clinical questions of interest. It leads to cross-functional discussions around different patient journeys and ensures that the protocol is clear on how different events occurring after the study medication start are handled. The framework, in turn, reduces unfocused analyses and, thus, risk.
Oncology is not specifically mentioned in ICH E9 (R1), and time-to-event endpoints are mentioned minimally. As such, various stakeholders have been working on the interpretation and implementation of the estimand framework in clinical trial practice for different therapeutic areas.
Approaching estimands for an oncology clinical trial
Regulatory authorities now expect estimands for all confirmatory trials, but they also consider them helpful for early development studies. Introducing estimands early on may help to identify in advance potential issues that could distort our interpretation of safety and efficacy (intercurrent events). The construction of estimands should begin early in the clinical development process and be used to plan the study design, data collection, statistical methods and sensitivity analyses (including handling of missing data). The primary estimand and overall strategy for handling intercurrent events should be defined in both the development synopsis and the protocol.
Estimands construction starts with bringing all stakeholders to the table for a structured dialogue. This is not just a discussion among statisticians, but rather a multi-disciplinary endeavor, bringing together diverse perspectives — potentially including collaboration with the regulatory agency — through a framework that structures discussions about the design, analysis and interpretation of oncology trials.
More specifically, an interdisciplinary team working on a Phase III oncology trial should agree upon an estimand that is precisely defined through five attributes (A to E below):
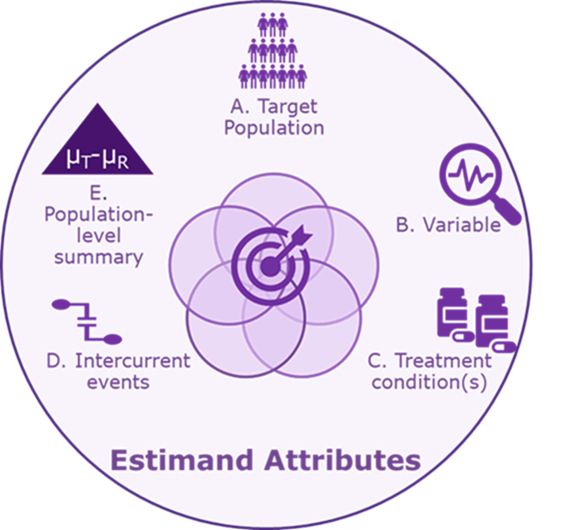
A. Target population: The population of all patients who would meet the study’s eligibility criteria (e.g., adult patients with a specific type of cancer).
B. Variable: The time from the start of treatment to progression or death.
C. Treatment condition(s): The interventional and control treatment (reference products or placebo) with dosing regimen and duration. These may be administered in addition to standard of care.
D. Intercurrent event(s): The strategy for handling events that can distort interpretation. Common intercurrent events in oncology trials are treatment discontinuation due to tolerability, death, treatment switching, dose adjustments or interruptions, start of a new cancer therapy, and surgery.
E. Population-level summary: Hazard ratio (Interventional treatment/control treatment) or median difference in survival times.
Intercurrent events impact interpretation of outcome
Interpreting the effect of a therapy is complicated by the different ways in which individual patients may respond to treatment. Some patients will tolerate a therapy and adhere to its administration schedule, while others will not. Some patients will require changes in dose or will start a new therapy, while others will not. In short, the diversity of the patient journey that is part of clinical practice, which includes unfavorable and often unavoidable situations, is also part of clinical trials (i.e., “intercurrent events”).
This is especially the case in oncology clinical trials, which tend to be long and complex and, thus, may have more opportunities for intercurrent events to occur. Failure to properly account for different intercurrent events may, in turn, lead to failure to demonstrate treatment effect and overall survival benefit.
As illustrated in the diagram below, differences in how a patient’s progression-free survival is interpreted can depend on the chosen strategies to handle intercurrent events (i.e., consider how the chosen strategies formulate the clinical question of interest).
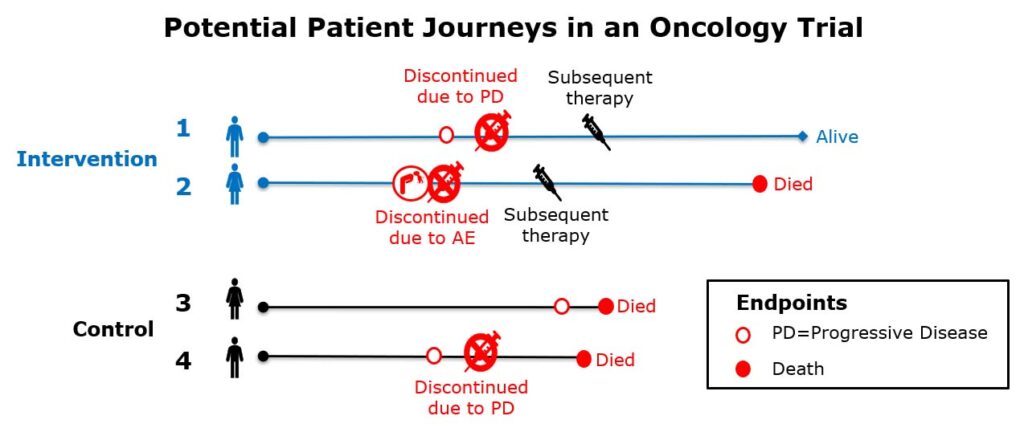
Clinical questions of interest are:
- Quantify the benefit to (progression-free) survival for those treated with the investigational drug compared to control
- How would the (progression-free) survival compare between patients treated with intervention versus control had they not received subsequent therapies?
How should subsequent therapies be handled in progression-free survival?
i. Progression-free survival regardless of whether a patient received subsequent therapy?
ii. Progression-free survival had patients not received subsequent therapies?
iii. Progression-free survival without any subsequent therapies or changes to standard of care medications?
As we can see in this simplified illustration, the results and interpretation of the outcome would be quite different depending on how you define this; specifically, subsequent therapies become part of the treatment condition being studied in (i) whereas (ii) considers subsequent therapy as a confounding factor to be removed from the treatment effect, and (iii) considers use of subsequent therapy as failure of the intervention. The point is that while there is no universally “correct” strategy, by aligning an interdisciplinary team around the same question of scientific interest — taking what might be a set of implicit assumptions and making them transparent — the estimand framework provides an alignment and focus to address the complexity introduced by diverse patient journeys defined by intercurrent events.
In this way, the estimand framework lends itself particularly well to oncology trials as it allows for formulation of precise scientific questions of interest with a prospective approach to handle intercurrent events.
Potential strategies to handle common oncology intercurrent events
There are multiple ways to incorporate intercurrent events into our scientific question of interest. The ICH E9 (R1) addendum outlines five strategies, clarifying that “whether or not the naming convention is used, it is required that the choice of strategy is unambiguously clear once the estimand is constructed.”
Below are three strategies that are particularly relevant for oncology studies presented with examples for intercurrent events that they might be suitable to handle.
Treatment policy: The occurrence of the intercurrent event is considered irrelevant
Hypothetical strategy: A scenario is envisaged in which the intercurrent event would not occur
Composite strategy: intercurrent event is considered to be informative about patient’s outcome and is therefore incorporated into definition of the variable
For example, the team will discuss the strategies that are appropriate for each intercurrent event and may conclude they wish to take a mix of three approaches (in estimands A, B and C).
| Estimand Label | A | B | C |
| Intercurrent Event Strategies | |||
| Discontinued interventional treatment | Treatment policy | Hypothetical | Composite |
| Subsequent therapy | Treatment policy | Hypothetical | Composite |
| Change to co-administered standard of care drugs | Treatment policy | Hypothetical | Composite |
| Unrelated death (from another cause unrelated to investigational product or disease) | Composite | Composite | Composite |
It is advisable to describe these estimands concisely and document in the protocol with the objectives.
Estimand A: Hazard ratio (investigational drug/reference drug) of progression or death from any cause in adult patients with <cancer type> irrespective of discontinuation of interventional treatment, start of subsequent anti-cancer therapy or changes in other standard of care medications.
Estimand B: Hazard ratio (investigational drug/reference drug) of progression or death from any cause in adult patients with <cancer type> prior to discontinuation of interventional treatment, subsequent anti-cancer therapy and prior to changes in other standard of care medications.
Estimand C: Hazard ratio (investigational drug/reference drug) of progression, discontinuation of interventional treatment, subsequent anti-cancer therapy, changes in standard of care therapy, or death from any cause in adult patients with <cancer type>.
Note that estimand A requires follow-up of patients after subsequent anti-cancer therapy or changes in other standard of care medications. If this type of estimand is of interest, then that has impacts on study conduct and, in this case, requires permission to follow patients even if they are no longer on study treatment; this approach may be more practical for overall survival than progression-free survival where scans are required to confirm progression. Thus, clearly defining estimands helps to understand whether scans are necessary after certain intercurrent events.
The trial conduct and estimands should be clear on how to handle intercurrent events. This, in turn, helps bring transparency in reporting meaningful treatment effects, informs clinical decision-making for both patients and physicians and ultimately may help smooth regulatory approvals.
Authors:
- Jürgen Hummel, executive director, statistical science
- Sue McKendrick, statistical science director
- Natalia Kan-Dobrosky, associate statistical science director
- Nikolay Stoyanov, senior director, clinical science, medical and scientific strategy
- Monica Hadi, director, research science, Evidera
The authors are members of our estimands working group, a cross-functional forum designed to facilitate collaboration and idea sharing to optimize study design, data collection, monitoring and analyses in the implementation of estimands.