Patient Data Dashboard
The PreclarusTM patient data dashboard accelerates access to data to more efficiently monitor patient safety throughout the course of a clinical trial. Clinical study teams, including medical and clinical reviewers, biostatisticians and data analyzers can use the patient data dashboard to perform detailed, real-time analysis of accumulating subject data.
This detailed analysis enables faster insights that improve clinical trial quality and efficiencies by:
- Offering greater timeline and cost efficiencies
- Creating actionable intelligence for faster insight and decision-making
- Improving safety and efficacy monitoring
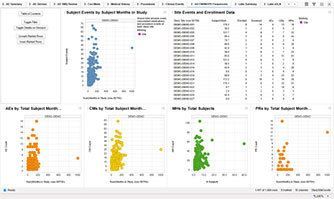
The data in the dashboard
The data is sourced in the FDA-recommended study data tabulation model (SDTM) and standardized and consolidated from multiple sources including:
• Electronic data capture/collection (EDC)
• Interactive voice response system (IVRS)
• Central lab database
